Kinetic Theory of Gases
Kinetic Theory of Gases: Overview
This topic covers concepts, such as, Avogadro's Number, Molar Mass, Molecules Obey Laws of Motion & Gas Laws from Kinetic Theory etc.
Important Questions on Kinetic Theory of Gases
If volume occupied by molecules is negligible, then what will be the pressure exerted by one mole of at is .
What is the average kinetic energy of molecules of an ideal gas leaking freely through an orifice of a container which has molecules at pressure in volume ?
A sample of an ideal gas occupies a volume at pressure and absolute temperature . The mass of each molecule is , then the density of the gas is
A stationary nucleus of a carbon atom is hit by a neutron in a nuclear reactor. If the collision is an elastic head-on collision, then find the fraction of kinetic energy transferred from the neutron to the carbon atom is . What is the value of ?
Which of the following is incorrect
In an artificial medium, air pressure and volume are related as where is a constant. Considering air as an ideal gas of density , then the speed of sound in air is
Under which of the following conditions is the law obeyed most closely by a real gas ?
A gas diffuse times as fast as hydrogen. Its molecular weight is
Number of in gram of water is?
If the intermolecular forces vanish away, the volume occupied by the molecules contained in water at standard temperature and pressure will be :-
One mole of ideal gas goes through process then change in temperature of gas when volume changes from to is:-
Two spherical vessels of equal volume, are connected by a narrow tube. The apparatus contains an ideal gas at one atmosphere and Now if one vessel is immersed in a bath of constant temperature and the other in a bath of constant temperature Then the common pressure will be
Gases obey vander-wall's equation at:
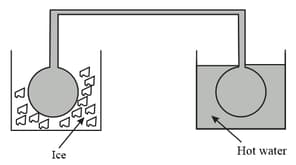
As shown in the above figure, two glass baths, one consisting of ice and other with hot water is taken. Now, two identical glass bulbs are interconnected by a thin glass tube at and filled with a gas. If one of the bulbs is dipped into an ice bath and other into the hot bath, then the pressure in the gas becomes times. What is the temperature of the hot bath in celsius?
A stationary nucleus of a carbon atom is hit by a neutron in a nuclear reactor. If the collision is an elastic head-on collision, then find the fraction of kinetic energy transferred from the neutron to the carbon atom:
A vessel of volume contains an ideal gas at room temperature and pressure . The gas is allowed to leak till its pressure falls to atmospheric pressure. Assuming that the process to be isothermal, the number of moles of the gas that have leaked are
(Consider , atmospheric pressure )
Let a gas be filled in a container such that the pressure in the container is . Now, if the is doubled and the mass of molecules is halved; then what is the final pressure in the container?
The number of molecules per unit volume of a gas depends on their distance r from the origin as, , where and are constants.The total number of molecules of the gas is proportional to:
An inverted bell, which is lying at the bottom of the lake deep, has of air trapped in it. The bell is brought to the surface of the lake then the volume of the trapped air will become (atmospheric pressure = and density of ):
